Diffuse Microglia Activation and Polio Virotherapy Outcome in Glioblastoma
Friday, April 21, 2023
.jpg)
Yuanfan Yang, MD, PhD (he/him/his)
Resident Physician
University of Alabama at Birmingham
Birmingham, Alabama, United States
ePoster Presenter(s)
Introduction: Cancer Immunotherapy with the highly attenuated rhino:poliovirus chimera, PVSRIPO, yielded long-term survival with durable responses in recurrent glioblastoma patients (Desjardins et al. NEJM, 2018). PVSRIPO targets host cells through the CD155 receptor, which is ectopically expressed in glioblastomas and naturally in mononuclear phagocytic cells, including glioma associated macrophage and microglia (GAMM). This study is to investigate the contributions of myeloid vs. neoplastic cells to polio virotherapy of malignant gliomas, and the mechanisms of immune therapy resistance to PVSRIPO.
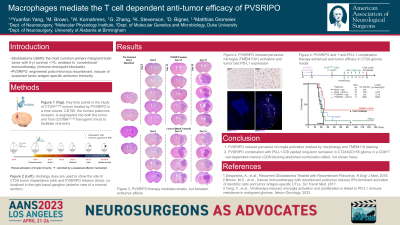
Methods: We optimized the immune-competent CT2A glioblastoma model for increased precision in tumor growth pattern, which made systemic neuropathological comparison of tissue responses to intra-tumoral PVSRIPO/control therapy possible. FFPE slides from tumor bearing brains (n=49) were collected in a time series. Activated microglia is defined by cells with rod-shaped nuclei without visible cytoplasm on H&E and Iba1+/Tmem119+ showing ramified processes on IHC staining. Multiplex IHC and RNA sequencing were performed to study the immune infiltrates and exhaustion markers over time. The role of the PD1:/PD-L1 axis in restricting the antitumor efficacy of PVSRIPO was tested using survival studies.
Results: PVSRIPO treatment caused intense engagement of the GAMM infiltrate associated with substantial, but transient tumor regression. This was accompanied by diffuse microglia activation and proliferation in ipsilateral brain parenchyma. Microglia activation also occurred in the absence of tumor cells, but not in wild type mice w/o CD155 receptor, demonstrating sub-lethal infection of microglia is required for PVSRIPO instigated immune activation. Anti-cancer immunity was later counterbalanced by a backdrop of sustained innate antiviral responses, associated with induction of the PD-L1 immune checkpoint on GAMM. Combining PVSRIPO with PD1/PD-L1 blockade led to durable remissions.
Conclusion : Our work implicates GAMM as active drivers of PVSRIPO induced antitumor inflammation and reveals profound and widespread neuroinflammatory activation of the CNS-resident myeloid compartment by PVSRIPO, which is closely associated with tumor regression.
Methods: We optimized the immune-competent CT2A glioblastoma model for increased precision in tumor growth pattern, which made systemic neuropathological comparison of tissue responses to intra-tumoral PVSRIPO/control therapy possible. FFPE slides from tumor bearing brains (n=49) were collected in a time series. Activated microglia is defined by cells with rod-shaped nuclei without visible cytoplasm on H&E and Iba1+/Tmem119+ showing ramified processes on IHC staining. Multiplex IHC and RNA sequencing were performed to study the immune infiltrates and exhaustion markers over time. The role of the PD1:/PD-L1 axis in restricting the antitumor efficacy of PVSRIPO was tested using survival studies.
Results: PVSRIPO treatment caused intense engagement of the GAMM infiltrate associated with substantial, but transient tumor regression. This was accompanied by diffuse microglia activation and proliferation in ipsilateral brain parenchyma. Microglia activation also occurred in the absence of tumor cells, but not in wild type mice w/o CD155 receptor, demonstrating sub-lethal infection of microglia is required for PVSRIPO instigated immune activation. Anti-cancer immunity was later counterbalanced by a backdrop of sustained innate antiviral responses, associated with induction of the PD-L1 immune checkpoint on GAMM. Combining PVSRIPO with PD1/PD-L1 blockade led to durable remissions.
Conclusion : Our work implicates GAMM as active drivers of PVSRIPO induced antitumor inflammation and reveals profound and widespread neuroinflammatory activation of the CNS-resident myeloid compartment by PVSRIPO, which is closely associated with tumor regression.
