An Institutional Approach to Incorporating Genomics and Epigenetic Testing in Clinical Neuro-Oncology Care
An Institutional Approach to Incorporating Genomics and Epigenetic Testing in Clinical Neuro-oncology Care
Friday, April 21, 2023

Patrick Halloran, BS
Medical Student
University of Connecticut
Farmington, Connecticut, United States
ePoster Presenter(s)
Introduction: There are currently limited treatment options for many central nervous system (CNS) tumor diagnoses. Improved understanding of the mechanisms of molecular tumorigenesis has revealed how these tumors arise and provided a catalog of genomic biomarkers and therapeutic targets to better stratify and treat patients. A cohesive incorporation of genetic techniques into routine clinical care can impact the outcomes for neuro-oncology patients.
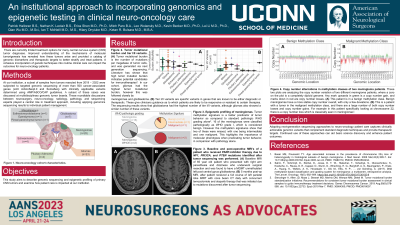
Methods: A subset of samples from tumors resected at our institution from 2019 – 2022 were subjected to targeted genomic sequencing of more than 500 cancer-associated genes (JAX ActionSeq2.0 and SomaSeq) with clinically applicable variants determined using AMP/ASCO/CAP guidelines. A subset of these cases was discussed at multidisciplinary genomic tumor boards. These roundtable discussions between neurosurgery, neuro-oncology, radiology, pathology, and sequencing experts played a central role in treatment approach, including applying genomics sequencing results to individual patient management.
Results: A total of 69 primary CNS tumors underwent targeted genomic sequencing, the majority being glioblastomas (n=7), other gliomas (n=23), and meningiomas (n=28). Glioblastomas carried the highest amount of clinically actionable (Tier I/II) variants (median = 4, range = 3-8), followed by gliomas (median = 3, range = 1-13) and meningiomas (median = 1, range = 0-3). Specific mutations identified on genetic sequencing elicited changes in neuro-oncology care in several of the patients. One example was genetic profiling of an occipital lobe glioblastoma resected from a 68-year-old male that demonstrated 8 clinically actionable variants including copy number losses of the DNA repair proteins BRCA2 and RAD51, which prompted PARP inhibitor (Olaparib) and bevacizumab therapy. Follow up MRI at 4 months demonstrated minimal tumor recurrence.
Conclusion : Adapting next-generation sequencing approaches to neuro-oncology patient care captures clinically actionable genomic variants that complement standard diagnostic techniques and provide therapeutic targets. Continued use of these approaches can aid basic science discovery and enhance patient outcomes.
Methods: A subset of samples from tumors resected at our institution from 2019 – 2022 were subjected to targeted genomic sequencing of more than 500 cancer-associated genes (JAX ActionSeq2.0 and SomaSeq) with clinically applicable variants determined using AMP/ASCO/CAP guidelines. A subset of these cases was discussed at multidisciplinary genomic tumor boards. These roundtable discussions between neurosurgery, neuro-oncology, radiology, pathology, and sequencing experts played a central role in treatment approach, including applying genomics sequencing results to individual patient management.
Results: A total of 69 primary CNS tumors underwent targeted genomic sequencing, the majority being glioblastomas (n=7), other gliomas (n=23), and meningiomas (n=28). Glioblastomas carried the highest amount of clinically actionable (Tier I/II) variants (median = 4, range = 3-8), followed by gliomas (median = 3, range = 1-13) and meningiomas (median = 1, range = 0-3). Specific mutations identified on genetic sequencing elicited changes in neuro-oncology care in several of the patients. One example was genetic profiling of an occipital lobe glioblastoma resected from a 68-year-old male that demonstrated 8 clinically actionable variants including copy number losses of the DNA repair proteins BRCA2 and RAD51, which prompted PARP inhibitor (Olaparib) and bevacizumab therapy. Follow up MRI at 4 months demonstrated minimal tumor recurrence.
Conclusion : Adapting next-generation sequencing approaches to neuro-oncology patient care captures clinically actionable genomic variants that complement standard diagnostic techniques and provide therapeutic targets. Continued use of these approaches can aid basic science discovery and enhance patient outcomes.
